Human embryo development and early organ formation remain largely unexplored due to ethical issues surrounding the use of embryos for research as well as limited availability of materials to study. In a paper published April 6 in the journal Cell Stem Cell, a team of investigators from China report for the first time the creation of embryo-like structures from monkey embryonic stem cells. The investigators also transferred these embryo-like structures into the uteruses of female monkeys and determined that the structures were able to implant and elicit a hormonal response similar to pregnancy.
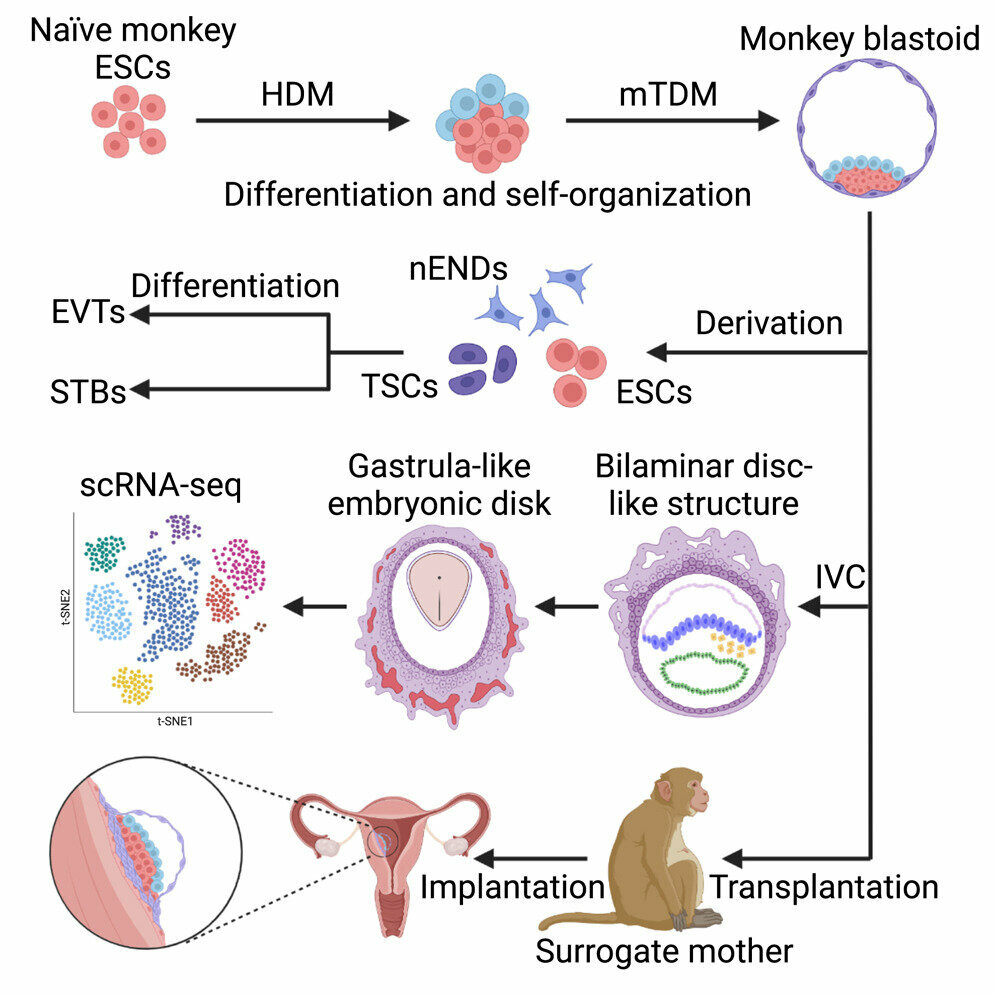
"The molecular mechanisms of human embryogenesis and organogenesis are largely unclear," says co-corresponding author Zhen Liu of the Chinese Academy of Sciences (CAS) in Shanghai. "Because monkeys are closely related to humans evolutionarily, we hope the study of these models will deepen our understanding of human embryonic development, including shedding light on some of the causes of early miscarriages."
"This research has created an embryo-like system that can be induced and cultured indefinitely," says co-corresponding author Qiang Sun, also of CAS. "It provides new tools and perspectives for the subsequent exploration of primate embryos and reproductive medical health."
The investigators started with macaque embryonic stem cells, which they exposed to a number of growth factors in cell culture. These factors induced the stem cells to form embryo-like structures for the first time using non-human primate cells.
When studied under a microscope, the embryo-like structures, also called blastoids, were found to have similar morphology to natural blastocysts. As they further developed in vitro, they formed arrangements that looked like the amnion and yolk sac. The blastoids also started to form the types of cells that eventually make up the three germ layers of the body. Single-cell RNA sequencing revealed that the different types of cells found within the structures had similar gene expression patterns to cells found in natural blastocysts or post-implantation embryos.
The blastoids were then transferred into the uteruses of 8 female monkeys; in 3 of the 8, the structures implanted. This implantation resulted in the release of progesterone and chorionic gonadotropin, hormones normally associated with pregnancy. The blastoids also formed early gestation sacs, fluid-filled structures that develop early in pregnancy to enclose an embryo and amniotic fluid. However, they did not form fetuses and the structures disappeared after about a week.
In future work, the investigators plan to focus on further developing the system of culturing embryo-like structures from monkey cells. "This will provide us with a useful model for future study," says co-corresponding author Fan Zhou of Tsinghua University. "Further application of monkey blastoids can help to dissect the molecular mechanisms of primate embryonic development."
The researchers acknowledge the ethical concerns surrounding this type of research but emphasize that there are still many differences between these embryo-like structures and natural blastocysts. Importantly, the embryo-like structures do not have full developmental potential. They note that for this field to advance it's important to have discussions between the scientific community and the public.
"This research has created an embryo-like system that can be induced and cultured indefinitely," says co-corresponding author Qiang Sun, also of CAS. "It provides new tools and perspectives for the subsequent exploration of primate embryos and reproductive medical health."
The investigators started with macaque embryonic stem cells, which they exposed to a number of growth factors in cell culture. These factors induced the stem cells to form embryo-like structures for the first time using non-human primate cells.
When studied under a microscope, the embryo-like structures, also called blastoids, were found to have similar morphology to natural blastocysts. As they further developed in vitro, they formed arrangements that looked like the amnion and yolk sac. The blastoids also started to form the types of cells that eventually make up the three germ layers of the body. Single-cell RNA sequencing revealed that the different types of cells found within the structures had similar gene expression patterns to cells found in natural blastocysts or post-implantation embryos.
The blastoids were then transferred into the uteruses of 8 female monkeys; in 3 of the 8, the structures implanted. This implantation resulted in the release of progesterone and chorionic gonadotropin, hormones normally associated with pregnancy. The blastoids also formed early gestation sacs, fluid-filled structures that develop early in pregnancy to enclose an embryo and amniotic fluid. However, they did not form fetuses and the structures disappeared after about a week.
In future work, the investigators plan to focus on further developing the system of culturing embryo-like structures from monkey cells. "This will provide us with a useful model for future study," says co-corresponding author Fan Zhou of Tsinghua University. "Further application of monkey blastoids can help to dissect the molecular mechanisms of primate embryonic development."
The researchers acknowledge the ethical concerns surrounding this type of research but emphasize that there are still many differences between these embryo-like structures and natural blastocysts. Importantly, the embryo-like structures do not have full developmental potential. They note that for this field to advance it's important to have discussions between the scientific community and the public.
Source from:
Cell Press
Cell Press
